Preguntas frecuentes sobre salas limpias modulares
P: ¿Qué es la tecnología de cuartos limpios?
R: La tecnología de cuartos limpios es el diseño, los componentes y el método de instalación utilizados para crear ambientes ultralimpios (cuartos limpios) para aplicaciones como la fabricación de semiconductores, productos farmacéuticos y dispositivos médicos. Los diferentes diseños incluyen cuartos limpios de recirculación, de un solo paso, modulares y de paredes blandas. Los componentes incluyen unidades de filtrado de ventiladores HEPA, paredes modulares, azulejos de techo para cuartos limpios, sistemas de esclusa, medidores magnahelic y luces para cuartos limpios. Los métodos de instalación incluyen cuartos limpios construidos en el sitio stick built (construcción estándar) y cuartos limpios modulares (prefabricados en planta y ensamblados en el sitio).
P: ¿Qué es un cuarto limpio portátil?
R: Portable cleanrooms are cleanrooms which can be moved without being disassembled. Smaller softwall cleanrooms can be mounted on casters and rolled to new locations inside same factory. It is possible to build a cleanroom on a 40x8 trailer and tow it to different locations.
P:¿Cuáles son las características y los beneficios de los cuartos limpios modulares?
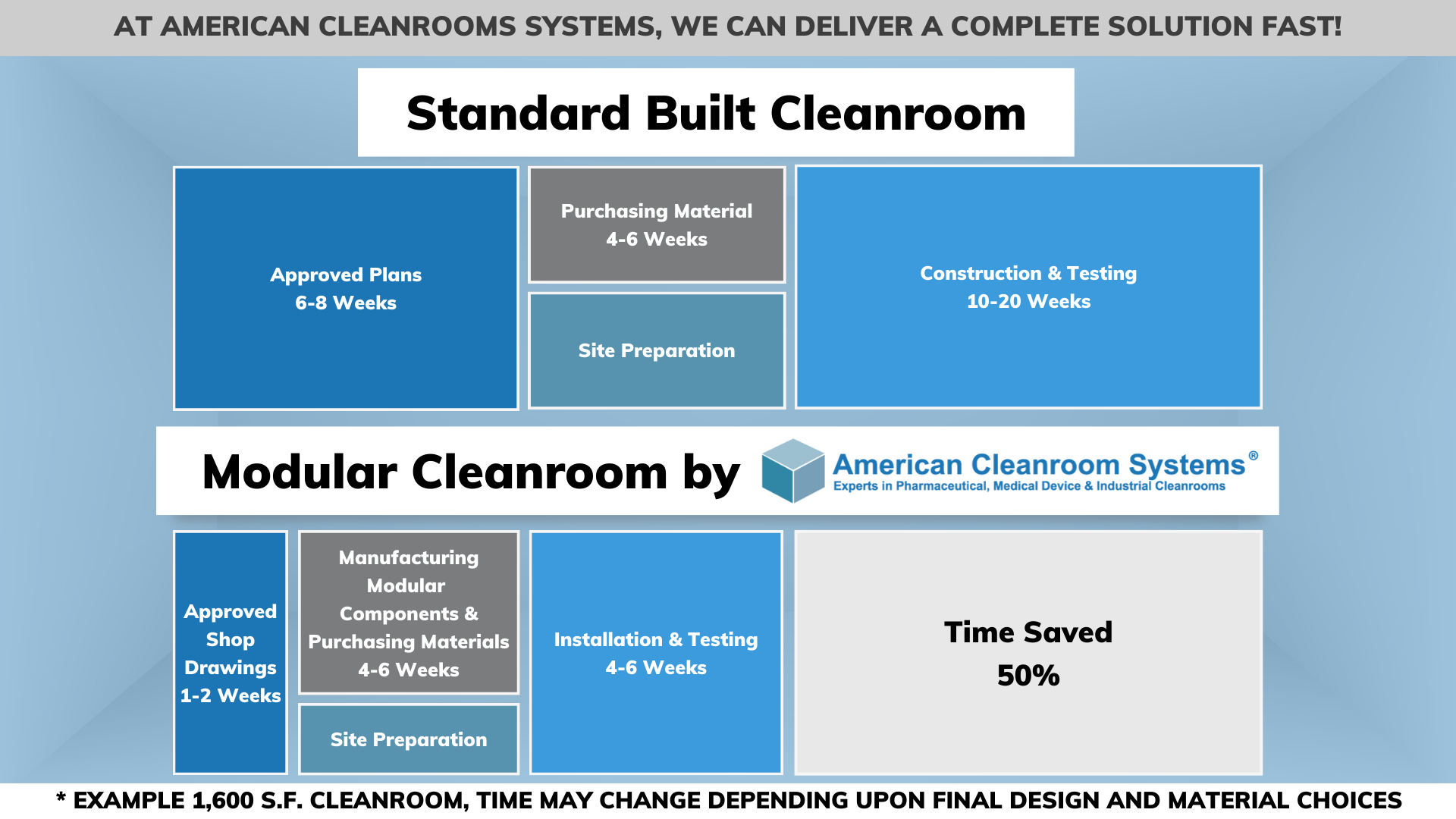
R: Modular cleanrooms offer all the same features of standard built cleanrooms (stick built). They can go from ISO-5 to ISO-8 (class 100-100k) with temperature and RH control. They can also have motorized sliding doors, roll up equipment doors, pass thru’s, gown rooms, heat welded vinyl flooring, windows, air conditioning, static dissipative walls, and chemical resistant walls. Modular cleanrooms can be used for all industries – pharmaceutical, medical device, industrial, defense, aerospace, semiconductor, food, E-liquid, CBD, and government. Cost is very similar to standard construction.
Benefits of using a modular cleanroom vs. standard construction include faster manufacture and installation. Another benefit is the ease of modification if the customer needs to increase the size, add more rooms, or upgrade cleanroom class. It is not uncommon to disassemble a modular cleanroom and reassemble it at another location. Some people classify the modular cleanroom as temporary equipment enclosures allowing them to bypass the cost and delay of building permits and also get favorable tax treatment.
P: ¿En qué industrias es más común cada tipo de cuarto limpio?
R: The semiconductor industry was one of the first industries to adopt cleanrooms. The extremely small feature size (less than 1 micron) on computer chips require class 100 cleanroom to achieve good manufacturing yield.
La industria farmacéutica es uno de los principales usuarios de los cuartos limpios para mantener un entorno estéril en la fabricación de medicamentos. Los requisitos de validación de la FDA y las CGMP exigen el uso de entornos controlados, como los cuartos limpios.
The medical device industry is also a major user of cleanrooms to maintain sterile environment for drug manufacturing. Like the pharmaceutical industry, the medical device industry is regulated by the FDA. CGMP requirements mandate the use of controlled environments such as cleanrooms.
The defense industry is a major user of cleanrooms. From precision electronics, lasers, solid state sensors to composite manufacturing, there are many applications that require cleanrooms for good manufacturing yield or accurate test measurements.
Otros lugares que utilizan cuartos limpios son los laboratorios de investigación y desarrollo, fábricas de medicamentos , fabricantes de nutracéuticos, procesadores de alimentos, empresas de e-líquidos, empresas de CBD, moldeo por inyección de plástico, industria aeroespacial y laboratorios de pruebas.
P: ¿Qué es un cuarto limpio móvil?
R: A mobile cleanroom is a cleanroom that is built into a trailer and can be moved to different locations. They are often used as temporary cleanrooms while the permanent cleanroom is being renovated. Small softwall cleanrooms mounted on casters are sometimes also called mobile cleanrooms.
P: ¿Cuáles son los requisitos de un cuarto limpio?
R: Most common cleanroom requirements include cleanroom classification, size, number of rooms, cleanroom flooring, cleanroom ceiling height, cleanroom chemical resistance, and cleanroom temperature and humidity.
P: ¿Cuáles son las diferentes clases de cuartos limpios?
R: There are both two cleanroom classification standards, ISO and FED 209E. ISO is per cubic meter and FED 209E is per cubic foot.
ISO-8/class 100,000 - This is the lowest cleanroom class. Requirements include 20 air changes per hour of HEPA filtered air and less the 700 particles per cubic foot greater than 5 microns.
ISO-7/class 10,000 - This is the 2nd lowest cleanroom class. Requirements include 60 air changes per hour of HEPA filtered air and less than 70 particles per cubic foot greater than 5 microns.
ISO-6/class 1000 - This is considered a high cleanroom class. Requirements include 180 air changes per hour of HEPA filtered air and less than 7 particles per cubic foot greater than 5 microns.
ISO-5/class 100 class 100 - This is considered the highest cleanroom class. Requirements include between 250 and 300 air changes per hour of HEPA filtered air and zero particles per cubic foot greater than 5 microns.
P: ¿Qué es un cuarto limpio prefabricado?
R: A modular cleanroom is often referred to as a pre-fabricated cleanroom because all the components of the cleanroom are custom manufactured in an off-site factory prior to installation. The components are shipped to a job site where they are assembled to create the modular cleanroom. This modular approach can reduce the time of manufacture and installation to 50% of standard construction.
P: ¿Cuáles son los componentes de un cuarto limpio?
R: Key components of a cleanroom include HEPA filtration, cleanroom walls, cleanroom plenum, cleanroom ceiling, cleanroom flooring, cleanroom lights, cleanroom doors, magnehelic gauges, interlocks, pass thrus, and air conditioning. Other possible components include cleanroom vinyl strip curtains, cleanroom intercoms, cleanroom exhaust system, humidifiers, and dehumidifiers.
P: ¿Cuáles son los componentes de un cuarto limpio modular?
R: Los componentes de un cuarto limpio modular pueden incluir: sistema de pared modular, plénum modular, sistema de techo de rejilla, unidades de filtro de ventilador HEPA, paredes de aire de retorno, iluminación y puertas.
P: ¿Cuáles son las aplicaciones de un cuarto limpio modular?
R: Las aplicaciones de los cuartos limpios modulares incluyen la fabricación de productos farmacéuticos, la fabricación de dispositivos médicos, la elaboración de productos farmacéuticos, la fabricación de semiconductores, investigación y desarrollo, laboratorios de pruebas, laboratorios de láser, moldeo por inyección de plástico, fabricación de mascarillas, fabricación de e-líquidos, salas de extracción de CBD, procesamiento de alimentos y nutracéuticos.
P: ¿Cuáles son las aplicaciones de un cuarto limpio prefabricado?
R: Prefabricated or modular cleanrooms are ideal when customer wants fast installation without sacrificing performance or cost. Other benefits that drive the selection of a prefabricated or modular cleanroom include easy expansion or modification and ability to relocate the cleanroom to a new location.
P: ¿Cómo se prepara la instalación de un cuarto limpio modular?
R: Prior to modular cleanroom installation, the area of install should be cleared. This means removing or raising any ducting, conduit, plumbing or sprinklers that may be in the way. Concrete slab should be patched, leveled and if needed, a moisture barrier installed. Any equipment in the area should be relocated.
P: ¿Cómo se prepara la instalación de un cuarto limpio prefabricado?
R: Prefabricated cleanroom means all the modular components are manufactured and cut to specific sizes prior to shipping from the factory. The area of cleanroom install should be clear and a concrete slab prepared for cleanroom flooring. Unlike standard construction, the assembly of a prefabricated cleanroom does not create significant dirt and can be completed much faster.
P: ¿Qué es un cuarto limpio en la industria farmacéutica?
R: Una sala limpia farmacéutica es una sala limpia que se utiliza para la fabricación de productos farmacéuticos.